2025-08-11 ノースウェスタン大学
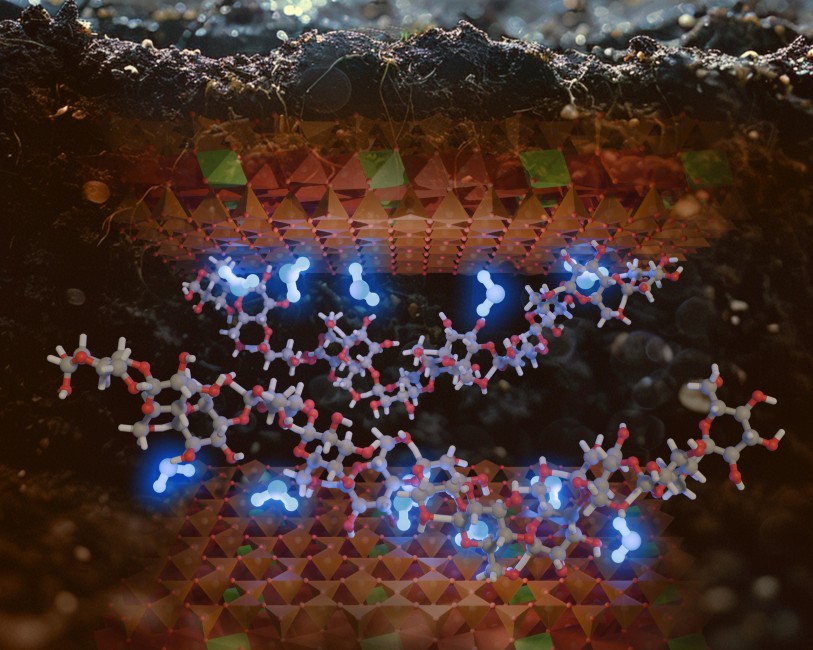 Illustration of water trapped as molecular bridges at carbohydrate-clay interfaces. Image by the Aristilde Research Group
Illustration of water trapped as molecular bridges at carbohydrate-clay interfaces. Image by the Aristilde Research Group
<関連情報>
- https://news.northwestern.edu/stories/2025/08/how-organic-matter-traps-water-in-soil-even-in-the-driest-conditions/
- https://academic.oup.com/pnasnexus/advance-article/doi/10.1093/pnasnexus/pgaf259/8229066
炭水化物と粘土の界面における水分保持のメカニズム Mechanisms of water retention at carbohydrate-clay interfaces
Sabrina E Kelch , Benjamin Barrios-Cerda , Yeonsoo Park , Eric Ferrage , Ludmilla Aristilde
PNAS Nexus Published:09 August 2025
DOI:https://doi.org/10.1093/pnasnexus/pgaf259
Abstract
Clay minerals are well documented to facilitate retention of water and organic matter in terrestrial soils, Martian regolith, and meteorites. Yet, the mechanisms underlying water trapping within these mineral-organic matter associations are poorly understood. Here, we investigate these mechanisms with montmorillonite, a smectite clay, populated with carbohydrates of different structures. By capturing relative proportion of bound versus freely exchangeable waters by mass spectrometry during thermogravimetric analysis, we observe up to 2.3-fold increase in bound waters in samples with adsorbed carbohydrates. Temperature-dependent carbon loss from adsorbed 13C-labeled carbohydrate reveals promoted organic carbon trapping at low moisture. We determine that the amount of this trapped carbon is correlated positively with the population of bound waters. Molecular dynamics simulations of a carbohydrate-populated clay nanopore identify different interfacial waters, involving direct single or multiple hydrogen bonds on the clay surface without or with simultaneous hydrogen bonding with adsorbed carbohydrates. Quantum mechanics-based computations highlight up to 5-fold greater binding energy for bound waters associated with adsorbed carbohydrates on the clay surface, compared to bound waters in the absence of carbohydrates. Thus, our experimental and theoretical results collectively reveal that interfacial waters bridging hydrated organic matter to the clay surface promote water trapping within mineral-organic associations.



